 Research Projects
Research Projects
We develop and combine quantitative microscopy and engineering techniques to gain knowledge of the structure, transport, thermodynamics, and mechanics of synthetic lipid bilayer membrane and monolayer systems. These serve as models of real biological membranes, and in some cases have technological applications that are easily identified (e.g. drug delivery devices). Here are some examples of recent research projects.
Functional Biomembrane Architectures in Mesoporous Materials
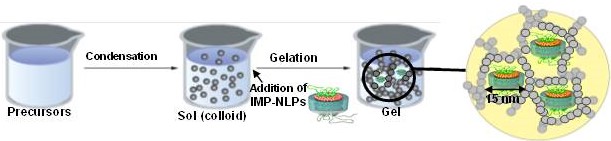 Entrapment of integral membrane proteins (IMPs) in transparent, nanoporous silica gels has proven to be a challenge, as current and previous techniques utilize liposomes as biological membrane hosts. The instability of liposomes in nanoporous gels is attributed to their size (~100 nm) and altered structure and lipid dynamics upon entrapment within the nanometer scale pores (5-50 nm) of silica gel. This ultimately results in disruption of protein activity. We intend to overcome these barriers by using nanolipoprotein particles (NLPs) as biomembrane hosts. NLPs are discoidal patches of lipid bilayer that are belted by amphiphilic scaffold proteins and have an average thickness of 5 nm, with diameters ranging from 10-15 nm. The IMP-NLP complexes are synthesized in a cell-free environment, which circumvents traditional protein reconstitution in membranes. To ensure proper functioning of the system, we have investigated the phase behavior of the lipids and the secondary structure of the scaffold proteins in gel-entrapped NLPs.
Entrapment of integral membrane proteins (IMPs) in transparent, nanoporous silica gels has proven to be a challenge, as current and previous techniques utilize liposomes as biological membrane hosts. The instability of liposomes in nanoporous gels is attributed to their size (~100 nm) and altered structure and lipid dynamics upon entrapment within the nanometer scale pores (5-50 nm) of silica gel. This ultimately results in disruption of protein activity. We intend to overcome these barriers by using nanolipoprotein particles (NLPs) as biomembrane hosts. NLPs are discoidal patches of lipid bilayer that are belted by amphiphilic scaffold proteins and have an average thickness of 5 nm, with diameters ranging from 10-15 nm. The IMP-NLP complexes are synthesized in a cell-free environment, which circumvents traditional protein reconstitution in membranes. To ensure proper functioning of the system, we have investigated the phase behavior of the lipids and the secondary structure of the scaffold proteins in gel-entrapped NLPs.
Domains/rafts in lipid bilayer membranes
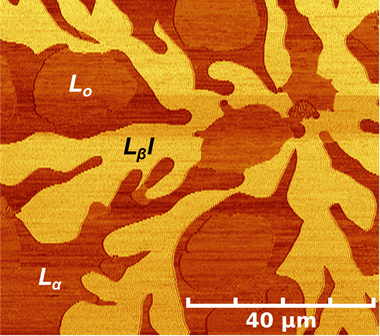 Cell membranes consist of a richly heterogeneous fluid mosaic of lipids and proteins. We investigate the physical mechanisms underlying observations in cell membranes by systematically studying multicomponent lipid bilayers that laterally separate into coexisting phases, or domains. We found that a high density of obstructions (domains) yielded low lipid diffusion coefficients and time-dependent (anomalous) diffusion behavior as observed in the highly obstructed environment of the cell membrane. Our group found that membranes reorganize to yield an asymmetric lipid distribution in opposing membrane leaflets except at the edges of lipid domains, explaining conflicting data in the literature as well as lending some thermodynamic justification for the dominant asymmetric distribution of cell membranes. We have measured the energy associated with lipid domain edges (line tension), a quantity of great interest to theorist in explaining membrane pattern formation and membrane raft behavior. In our approach, we observed, by AFM, nucleation of nanometer-scale lipid domains as a function of cholesterol content and applied nucleation theory to determine line tensions. Knowledge of chemical composition of cell membranes at the 10s to 100s of nm scale is out of the reach of current imaging technologies, yet it is critically important for answering questions about membrane organization and cell function. Our group, in an effort lead by Steve Boxer at Stanford and in collaboration with Ian Hutcheon at LLNL, took the next step in biomembrane imaging, using Nano Secondary Ion Mass Spectrometry to map the chemical composition of a phase-separating binary mixture of lipids developed by our laboratory with 70 to 100 nm resolution. Our most recent focus is in studying the impact of curvature and short-chain alcohols on these multicomponent lipid bilayers, a topic of biological and technological significance.
Cell membranes consist of a richly heterogeneous fluid mosaic of lipids and proteins. We investigate the physical mechanisms underlying observations in cell membranes by systematically studying multicomponent lipid bilayers that laterally separate into coexisting phases, or domains. We found that a high density of obstructions (domains) yielded low lipid diffusion coefficients and time-dependent (anomalous) diffusion behavior as observed in the highly obstructed environment of the cell membrane. Our group found that membranes reorganize to yield an asymmetric lipid distribution in opposing membrane leaflets except at the edges of lipid domains, explaining conflicting data in the literature as well as lending some thermodynamic justification for the dominant asymmetric distribution of cell membranes. We have measured the energy associated with lipid domain edges (line tension), a quantity of great interest to theorist in explaining membrane pattern formation and membrane raft behavior. In our approach, we observed, by AFM, nucleation of nanometer-scale lipid domains as a function of cholesterol content and applied nucleation theory to determine line tensions. Knowledge of chemical composition of cell membranes at the 10s to 100s of nm scale is out of the reach of current imaging technologies, yet it is critically important for answering questions about membrane organization and cell function. Our group, in an effort lead by Steve Boxer at Stanford and in collaboration with Ian Hutcheon at LLNL, took the next step in biomembrane imaging, using Nano Secondary Ion Mass Spectrometry to map the chemical composition of a phase-separating binary mixture of lipids developed by our laboratory with 70 to 100 nm resolution. Our most recent focus is in studying the impact of curvature and short-chain alcohols on these multicomponent lipid bilayers, a topic of biological and technological significance.
Lipid monolayer stabilized micron-scale bubbles
 Micron-scale gas-in-liquid bubbles (microbubbles) can be stabilized by a thin shell composed primarily of lipids with medical applications such as FDA approved ultrasound contrast agents, blood substitutes, and targeted drug and gene delivery vehicles. We have utilized unique characteristics of these tiny microbubbles to discover a richness of physical chemical behavior in microbubble shells closely resembling the lipid shells of medical microbubbles. While studying gas diffusion in microbubbles, our group developed a unifying equation for gases crossing a condensed nanometer-scale film that included the impact of defects, a mechanism that is analogous to gas diffusion through interstitial sites in a crystalline lattice. By observing collapse of the lipid shell in shrinking microbubbles, our group deduced that in thin films, the mechanism of collapse depends upon both reduced temperature and area compression rate, a consequence of time-temperature superposition. We elucidated discrepancies in the conclusions drawn from many studies of binary lipid-lipopolymer mixtures used to coat microbubbles and liposomal drug delivery devices by developing complete surface pressure-composition phase diagrams and discovering a stabilized condensed stoichiometric 3:1 lipid:lipopolymer complex. Our group, in collaboration with Paul Dayton of UC Davis and University of North Carolina, began the recent charge to use microfluidics to form monodisperse-sized microbubbles, and holds the record on the smallest sized (1 micrometer) microbubbles successfully coated with lipids in a microfluidics device. Ultrasound contrast is optimized in these mono-sized microbubbles because the resonance frequency of all the microbubbles matches the ultrasound bandwidth. This is currently not the case in FDA approved products. Our most recent focus is on a novel method to equip these microbubbles with vesicles for drug delivery.
Micron-scale gas-in-liquid bubbles (microbubbles) can be stabilized by a thin shell composed primarily of lipids with medical applications such as FDA approved ultrasound contrast agents, blood substitutes, and targeted drug and gene delivery vehicles. We have utilized unique characteristics of these tiny microbubbles to discover a richness of physical chemical behavior in microbubble shells closely resembling the lipid shells of medical microbubbles. While studying gas diffusion in microbubbles, our group developed a unifying equation for gases crossing a condensed nanometer-scale film that included the impact of defects, a mechanism that is analogous to gas diffusion through interstitial sites in a crystalline lattice. By observing collapse of the lipid shell in shrinking microbubbles, our group deduced that in thin films, the mechanism of collapse depends upon both reduced temperature and area compression rate, a consequence of time-temperature superposition. We elucidated discrepancies in the conclusions drawn from many studies of binary lipid-lipopolymer mixtures used to coat microbubbles and liposomal drug delivery devices by developing complete surface pressure-composition phase diagrams and discovering a stabilized condensed stoichiometric 3:1 lipid:lipopolymer complex. Our group, in collaboration with Paul Dayton of UC Davis and University of North Carolina, began the recent charge to use microfluidics to form monodisperse-sized microbubbles, and holds the record on the smallest sized (1 micrometer) microbubbles successfully coated with lipids in a microfluidics device. Ultrasound contrast is optimized in these mono-sized microbubbles because the resonance frequency of all the microbubbles matches the ultrasound bandwidth. This is currently not the case in FDA approved products. Our most recent focus is on a novel method to equip these microbubbles with vesicles for drug delivery.
